
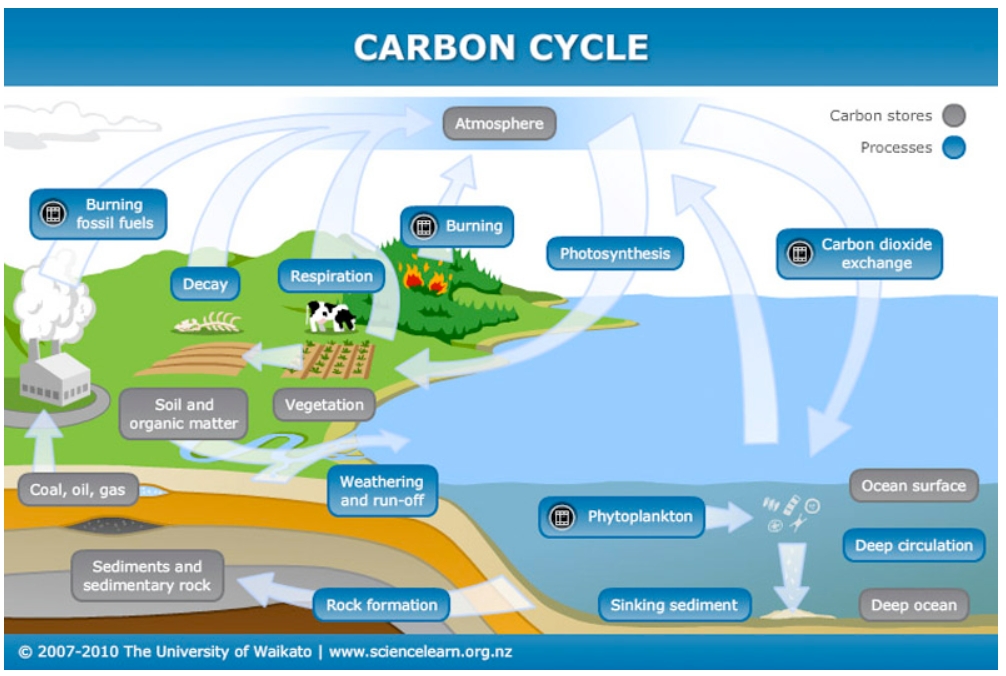
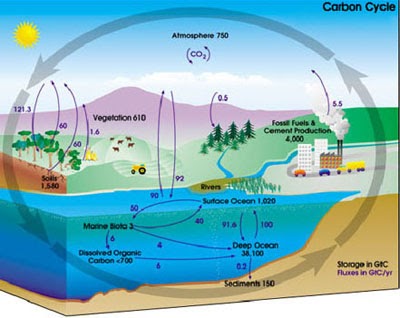
The ocean's acidity does rise with increased CO2, but the slow pace of ocean circulation prevents this process from developing useful momentum. These shells must dissolve in ocean water in order to be available to aid in the uptake of CO2, but the rate at which they dissolve is controlled by the ocean's acidity. One is the availability of carbonate, which comes from huge deposits of calcite (shells) in the upper levels of the ocean. However, once dissolved in the ocean, a carbon atom will stay there, on average, more than 500 years, estimates Michael McElroy, Butler professor of environmental science.īesides the slow pace of ocean turnover, two more factors determine the rate at which the seas take up carbon dioxide. This process takes place at an extremely low rate, measured in hundreds to thousands of years. The ocean absorbs CO2 from the atmosphere in an attempt to reach equilibrium by direct air-to-sea exchange. Understanding these natural mechanisms is important in forecasting the rise of atmospheric CO2 because even though plants and bodies of water now absorb surplus greenhouse gas, they could become new trouble spots. Of all the carbon dioxide (CO2) emitted into the atmosphere, one quarter is taken up by land plants, another quarter by the oceans.


 0 kommentar(er)
0 kommentar(er)
